Neon
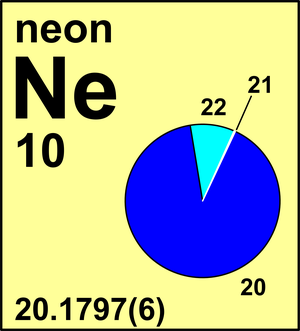
| Isotope | Atomic mass (Da) | Isotopic abundance (amount fraction) |
|---|---|---|
| 20Ne | 19.992 440 18(1) | 0.9048(3) |
| 21Ne | 20.993 8467(3) | 0.0027(1) |
| 22Ne | 21.991 3851(1) | 0.0925(3) |
In 1961, the Commission recommended Ar(Ne) = 20.183 based on gas-density measurements. At that time, the only reported measurements by mass spectrometry were not calibrated and were not considered to be reliable enough to serve as the basis for the atomic weight. In 1967, the Commission evaluated the results of two new calibrated mass spectrometric measurements and recommended Ar(Ne) = 20.179(3). In 1985, the Commission reduced the uncertainty of the standard atomic weight further and recommended Ar(Ne) = 20.1797(6) by combining the earlier results with new calibrated measurements. The atomic weight and its uncertainty refer to atmospheric Ne.
The annotation "g" refers to occurrences of Ne with diverse and anomalous isotopic compositions in some minerals and natural gases, derived in part from earth's mantle and from various nuclear reactions such as 18O(α,n)21Ne and 25Mg(n,α)22Ne.
© IUPAC 2003
CIAAW
Neon
Ar(Ne) = 20.1797(6) since 1985
The name derives from the Greek neos for "new". It was discovered from its bright orange spectral lines
by the Scottish chemist William Ramsay and the English chemist Morris William Travers in 1898 from
a liquefied air sample.


