Strontium
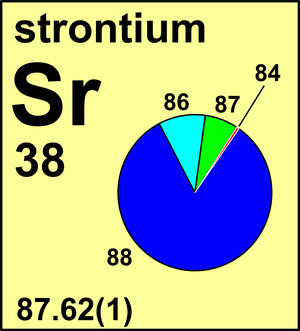
| Isotope | Atomic mass (Da) | Isotopic abundance (amount fraction) |
|---|---|---|
| 84Sr | 83.913 419(8) | 0.0056(2) |
| 86Sr | 85.909 260 73(4) | 0.0986(20) |
| 87Sr | 86.908 877 50(3) | 0.0700(20) |
| 88Sr | 87.905 612 26(4) | 0.8258(35) |
In its 1961 report, the Commission recommended Ar(Sr) = 87.62 based on the mass-spectrometric determination of Nier. This value was revised to Ar(Sr) = 87.62(1) in 1969 and it remains unchanged since.
Known natural variations in the abundance of 87Sr, the product isotope of radioactive 87Rb decay, prevent the recommendation of a more precise standard atomic-weight value. The n(87Sr)/n(86Sr) ratio is a convenient measure of that variability. Anomalous traces of almost pure 87Sr have been reported from rubidium ores, for which the atomic weight will not be in the tabulated standard atomic weight range.
The appreciable variability in n(87Sr)/n(86Sr) is the basis for Rb-Sr geochronology, and for analysis of source components in mixtures of water and geologic materials.
© IUPAC 2003
CIAAW
Strontium
Ar(Sr) = 87.62(1) since 1969
The name derives from Strontian, a town in Scotland. The mineral strontianite is found in mines in
Strontian. The element was discovered in 1792 by the Scottish chemist and physician Thomas Charles
Hope, who observed the brilliant red flame colour of strontium. It was first isolated by the English
chemist Humphry Davy in 1808.
Isotopic reference materials of strontium.


