Antimony
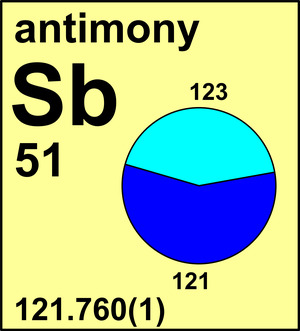
| Isotope | Atomic mass (Da) | Isotopic abundance (amount fraction) |
|---|---|---|
| 121Sb | 120.903 81(2) | 0.5721(5) |
| 123Sb | 122.904 21(1) | 0.4279(5) |
In the early 1960s, the Commission assessed that an average value of Ar(Sb) from chemical determinations was 121.75, whereas mass-spectrometric determinations yielded 121.76. The chemical value was evidently given a preference when in 1969 the Commission recommended Ar(Sb) = 121.75(3).
In 1989, the Commission adopted Ar(Sb) = 121.757(3) based on new mass-spectrometric measurements. This estimate was further revised to Ar(Sb) = 121.760(1) in 1993, based on more calibrated mass-spectrometric determinations.
Although no evidence of isotope fractionation of antimony in any of the terrestrial materials has been found, the "g" annotation is due to isotopic anomalies identified in the Oklo uranium deposit at Gabon, south-west Africa.
© IUPAC 2003
CIAAW
Antimony
Ar(Sb) = 121.760(1) since 1993
The name derives from the Greek, anti + monos for "not alone" or "not one" because it was found in many
compounds. The symbol Sb comes from stibium, which is derived from the Greek stibi for
"mark" because it was used for blackening eyebrows and eyelashes. The minerals stibnite (Sb2S3) and
stibine (SbH3) are two of more than one hundred mineral species, which were known in the ancient world.


