Argon
| Isotope | Atomic mass (Da) | Isotopic abundance (amount fraction) |
|---|---|---|
| 36Ar | 35.967 5451(2) | [0.0000, 0.0207] |
| 38Ar | 37.962 732(2) | [0.000, 0.043] |
| 40Ar | 39.962 383 12(2) | [0.936, 1.000] |
The atomic weight of argon is based on analyses of argon separated from air. In 1961, the Commission changed the recommended value of Ar(Ar) from 39.944, based on gas density measurements, to 39.948, based on the calibrated mass-spectrometric measurements reported by Nier. In 1979, the Commission examined the available literature and recommended Ar(Ar) = 39.948(1). This value of Ar(Ar) was one of the critical parameters to determine the value of the universal gas constant R by acoustic methods.
In 2007, the Commission recommended value for the isotope-amount ratio of n(40Ar)/n(36Ar) in air, which is of importance in geochronology and geochemistry, as 298.56(31).
The isotopic composition and atomic weight of argon are variable in terrestrial materials. Those variations are a source of uncertainty in the assignment of standard properties for argon,
but they provide useful information in many areas of science. Variations in the stable isotopic composition and atomic weight of argon are caused by several different processes, including
(1) isotope production from other elements by radioactive decay (radiogenic isotopes) or other nuclear transformations (e.g., nucleogenic isotopes), and
(2) isotopic fractionation by physical-chemical processes such as diffusion or phase equilibria.
Physical-chemical processes cause correlated mass-dependent variations in the argon isotope-amount ratios (40Ar/36Ar and 38Ar/36Ar),
whereas nuclear transformation processes cause mass-independent variations. While atmospheric argon can serve as an abundant and homogeneous isotopic reference, deviations from the atmospheric isotopic ratios in other argon occurrences limit the precision with which a standard atomic weight can be given for argon.
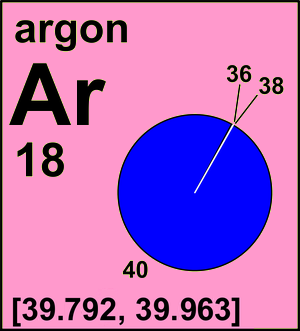
Published data indicate variation of argon atomic weights in normal terrestrial materials between 39.792 and 39.963. The upper bound of this interval is given by the atomic mass of 40Ar, as some samples contain almost pure radiogenic argon-40.
The lower bound is derived from analyses of pitchblende (uranium mineral) containing large amounts of nucleogenic 36Ar and 38Ar.
Within this interval, measurements of different isotope ratios (40Ar/36Ar or 38Ar/36Ar) at various levels of precision are widely used for studies in geochronology, water–rock interaction, atmospheric evolution, and other fields.
Radiogenic 40Ar is produced (along with 40Ca) by decay of a minor isotope of potassium (40K), which has a total half-life of 1.26(1) Ga. This radioactivity results in many geological samples having anomalous amounts of 40Ar and is the basis of the K-Ar and Ar-Ar dating methods used in geochronology. Samples containing only minor components of noble gases from non-radiogenic sources may have Ar(Ar) values approaching that of pure 40Ar. Owing to the wide distribution of potassium, even major sources of Ar such as some natural gas deposits and geothermal reservoirs can have sufficiently high 40Ar concentrations.
In contrast, it is much less common for natural samples to have n(40Ar)/n(36Ar) ratios significantly less than that of air. Radiogenic 36Ar can accumulate by decay of 36Cl (half-life = 0.301(2) Ma), which in turn is produced from 35Cl by neutron capture associated with cosmic-ray interactions in the atmosphere and with U and Th decay in the solid earth. Similarly, 38Ar may accumulate as a result of reactions such as 37Cl(n,γ)38Cl or 35Cl(α,p)38Cl. Some samples of argon extracted from microscopic Cl-bearing inclusions in minerals have been reported to have anomalously high concentrations of 36Ar and 38Ar that may be attributable to nucleogenesis.
Radioactive 37Ar and 39Ar are formed continuously in the atmosphere as products of cosmic-ray reactions, and they are components of cosmic dust entering the earth's atmosphere. Both isotopes also are formed by nuclear reactions on and beneath the earth's surface. At the present time, most of the new 39Ar introduced to the atmosphere each year is from nuclear reactors. 39Ar decays to 39K with a half-life of 269 a; while 37Ar decays to 37Cl with a half-life of 35 days. The amounts of 37Ar and 39Ar in normal samples are variable and may be useful in environmental studies, but they are several orders of magnitude too small to affect the standard atomic weight of argon at its current level of reported uncertainty.
Variation in the terrestrial isotopic composition and atomic weight of argon by J.K. Böhlke. Pure Appl. Chem. 2014 (86) 1421-1432
CIAAW
Argon
Ar(Ar) = [39.792, 39.963] since 2017
The name derives from the Greek argos for "lazy" or "inactive" because it does not combine with other
elements. It was discovered in 1894 by the Scottish chemist William Ramsay and the English physicist
Robert John Strutt (Lord Rayleigh) in liquefied air. Rayleigh's initial interest derived from a problem
posed by the English physicist Henry Cavendish in 1785, i.e., when oxygen and nitrogen were removed
from air, there was an unknown residual gas remaining.
Natural variations of argon isotopic composition
For more information about the natural variations of the atomic weight of argon please read IUPAC Technical Report Variation in the terrestrial isotopic composition and atomic weight of argon  by J.K. Böhlke. Pure Appl. Chem. 86, 1421-1432 (2014).
by J.K. Böhlke. Pure Appl. Chem. 86, 1421-1432 (2014).


