Calcium
| Isotope | Atomic mass (Da) | Isotopic abundance (amount fraction) |
|---|---|---|
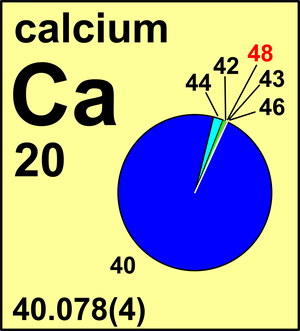
| 40Ca | 39.962 5909(2) | 0.969 41(156) |
| 42Ca | 41.958 618(1) | 0.006 47(23) |
| 43Ca | 42.958 766(2) | 0.001 35(10) |
| 44Ca | 43.955 482(2) | 0.020 86(110) |
| 46Ca | 45.953 69(2) | 0.000 04(3) |
| 48Ca | 47.952 5229(6) | 0.001 87(21) |
In 1983, the Commission with its liberalized policy on uncertainties, was able to recommend as standard atomic weight Ar(Ca) = 40.078(4) weighted toward the mass-spectrometric measurements. Moreover, the stated uncertainty includes all chemical, x-ray, and mass-spectrometric measurements believed to be significant by the Commission, as enumerated in its 1983 report.
There is evidence for minor isotope fractionation of calcium in Nature, causing variability of Ar(Ca) in normal sources that is within the uncertainty of the standard atomic weight. Variations in n(44Ca)/n(40Ca) can be reported as δ44Ca values relative to the calcium carbonate reference material NIST-SRM 915a. A recent compilation yielded a range of published δ44Ca values in natural samples from a low of -2.17 ‰ in a cougar bone with Ar(Ca) = 40.0778 to a high of +2.76 ‰ in egg white with Ar(Ca) = 40.0784. Elemental calcium with as δ44Ca = -6.0 ‰ (Ar(Ca) = 40.0773) also has been reported. Variations in the isotopic composition of marine calcium have occurred over the last 80 Ma.
In addition, there are many reports of anomalous isotopic composition of some minor samples of Ca, some of which may have arisen from the decay of 40K to 40Ca. The annotation "g" is therefore maintained for this element. 41Ca is an extinct radioisotope (with a half-life of 0.1 Ma), which can be used to date the early history of the solar system through its decay to 41K.
© IUPAC 2003
CIAAW
Calcium
Ar(Ca) = 40.078(4) since 1983
The name derives from the Latin calx for "lime" (CaO) or "limestone" (CaCO3) in which it was found.
It was first isolated by British chemist Humphry Davy in 1808 with help from the Swedish chemist Jöns
Jacob Berzelius and the Swedish court physician M. M. af Pontin.
Isotopic reference materials of calcium.


