Cerium
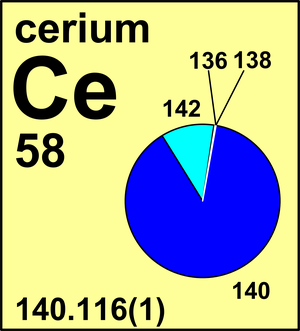
| Isotope | Atomic mass (Da) | Isotopic abundance (amount fraction) |
|---|---|---|
| 136Ce | 135.907 129(3) | 0.001 85(2) |
| 138Ce | 137.905 99(3) | 0.002 51(2) |
| 140Ce | 139.905 45(1) | 0.884 50(51) |
| 142Ce | 141.909 25(2) | 0.111 14(51) |
In 1961, the Commission recommended Ar(Ce) = 140.12 based on the average value of the mass-spectrometric measurements, which were in good agreement with earlier chemical determinations. The atomic weight and uncertainty of cerium were changed to their current values in the 1995 report of the Commission, based on new isotope-abundance data.
138Ce and 140Ce are the decay products of long-lived minor isotopes 138La and 144Nd, respectively. They have a negligible effect on Ar(Ce) in normal sources, but add justification to the "g" annotation, which also refers to the Oklo occurrence in Gabon, south-west Africa.
© IUPAC 2003
CIAAW
Cerium
Ar(Ce) = 140.116(1) since 1995
The name derives from the planetoid Ceres, which was discovered by the Italian astronomer Giuseppe
Piazzi in 1801 and named for Ceres, the Roman goddess of agriculture and harvest. Two years later, the
element cerium was discovered by the German chemist Martin-Heinrich Klaproth, who called it ochroeite earth
because of its yellow colour.
Cerium was independently discovered at the same time by the Swedish chemist
Jöns Jacob Berzelius and the Swedish mineralogist Wilhelm von Hisinger, who called it ceria. It was first
isolated in 1875 by the American mineralogist and chemist William Frances Hillebrand and the
American chemist Thomas H. Norton.


