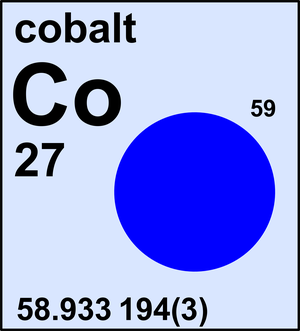
Cobalt
| Isotope | Atomic mass (Da) | Isotopic abundance (amount fraction) |
|---|---|---|
| 59Co | 58.933 194(3) | 1 |
Cobalt is a monoisotopic element and its atomic weight is determined solely by its isotope 59Co. The Commission last revised the standard atomic weight of cobalt in 2017 based on the latest Atomic Mass Evaluation by IUPAP.
59Co is readily transformed to 60Co, which decays to 60Ni with a half-life of 5.272 a. 60Co therefore serves as an extremely reproducible standard for radiation exposure.
© IUPAC 2003
CIAAW
Cobalt
Ar(Co) = 58.933 194(3) since 2017
The name derives from the German Kobold for "evil spirits" or "goblins", who were superstitiously
thought to cause trouble for miners because the mineral contained arsenic that injured their health and
the metallic ores did not yield metals when treated with the normal methods. Cobalt was discovered in 1735
by the Swedish chemist Georg Brandt.


