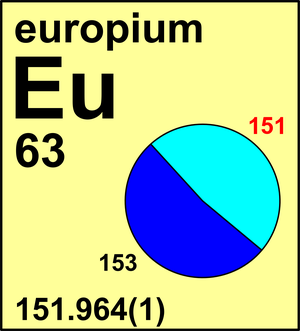
Europium
| Isotope | Atomic mass (Da) | Isotopic abundance (amount fraction) |
|---|---|---|
| 151Eu | 150.919 857(9) | 0.4781(6) |
| 153Eu | 152.921 237(9) | 0.5219(6) |
In 1961, the Commission recommended the atomic weight of europium to be Ar(Eu) = 151.96 based on recent mass-spectrometric determinations. Owing to the fact that europium has only two stable isotopes, in 1969, the standard atomic weight was assessed as Ar(Eu) = 151.96(1). Since that time, several another confirmatory isotopic composition measurements have become available and in 1995 the Commission recommended Ar = 151.964(1).
Natural variability of europium isotopes has not been studied and reported in the literature, however, the "g" footnote arises from the presence of naturally occurring fission products found in fossil reactors at Gabon, south-west Africa.
© IUPAC 2003
CIAAW
Europium
Ar(Eu) = 151.964(1) since 1995
The name derives from the continent of Europe. It was separated from the mineral samaria in magnesium-
samarium nitrate by the French chemist Eugène-Anatole Demarçay in 1896. It was also first isolated
by Demarçay in 1901.