Indium
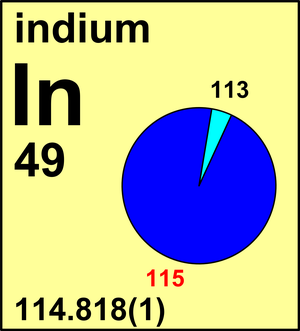
| Isotope | Atomic mass (Da) | Isotopic abundance (amount fraction) |
|---|---|---|
| 113In | 112.904 060(2) | 0.042 81(52) |
| 115In | 114.903 878 77(8) | 0.957 19(52) |
In 1991, the Commission changed the recommended value for the atomic weight of indium to Ar(In) = 114.818(3) based on high precision measurements of the metal and its compounds. The new measurement represents a significant improvement in the precision of the atomic weight and is in agreement with the previous values. The Commission revised the standard atomic weight of indium in 2011 in light of new mass-spectrometric measurements.
115In is β– active with a half-life so long, 441(25)×1015 a, that it neither affects Ar(In) nor has it given rise to recognized abnormal occurrences of tin.
© IUPAC 2003
CIAAW
Indium
Ar(In) = 114.818(1) since 2011
The name derives from the term "indigo" for the indigo-blue line in the element's spark spectrum. It
was discovered in 1863 by the German physicist Ferdinand Reich and the German metallurgist
Hieronymus Theodor Richter, while examining zinc blende. They isolated indium in 1867.


