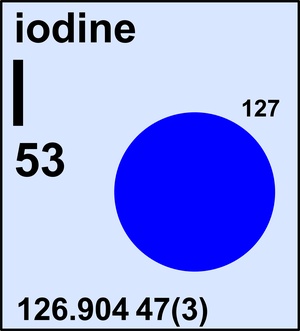
Iodine
| Isotope | Atomic mass (Da) | Isotopic abundance (amount fraction) |
|---|---|---|
| 127I | 126.904 47(3) | 1 |
Iodine is a monoisotopic element and its atomic weight is determined solely by its isotope 127I. The Commission last revised the standard atomic weight of iodine in 1985 based on the latest Atomic Mass Evaluation by IUPAP.
129I has been measured in terrestrial samples that have been exposed to cosmic radiation, and in materials that contain fallout from nuclear explosions. These measurements can be used for geochronological and environmental studies, but they also confirm the low abundance of 129I in nature, and its insignificance with respect to the atomic weight of iodine.
© IUPAC 2003
CIAAW
Iodine
Ar(I) = 126.904 47(3) since 1985
The name derives from the Greek iodes for "violet" because of its violet vapours. Iodine was discovered in
seaweed by the French chemist Bernard Courtois in 1811, and named by the French chemist Louis-Joseph Gay-Lussac, when he proved it was an element in 1814.