Iridium
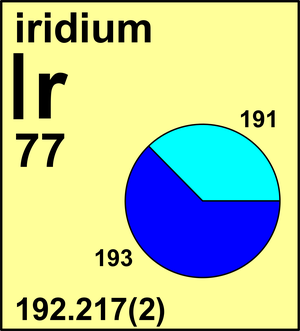
| Isotope | Atomic mass (Da) | Isotopic abundance (amount fraction) |
|---|---|---|
| 191Ir | 190.960 591(9) | 0.3723(9) |
| 193Ir | 192.962 924(9) | 0.6277(9) |
In 1969, the Commission recommended Ar(Ir) = 190.22(3) based on a closer analysis of earlier mass-spectrometric determinations. In 1993, the Commission changed the recommended value for the standard atomic weight of iridium to Ar(Ir) = 192.217(3) based on recent high-precision measurements using both positive and negative thermal ionization mass spectrometry. This value was reaffirmed in 2017 and represents a significant improvement in the precision of the atomic weight and is in agreement with the previous measurements.
© IUPAC 2003
CIAAW
Iridium
Ar(Ir) = 192.217(2) since 2017
The name derives from the Latin Iris, the Greek goddess of rainbows, because of the variety of colours
in the element's salt solutions. Iridium and osmium were both discovered in a crude platinum ore in
1803 by the English chemist Smithson Tennant. Iridium was discovered independently by the French
chemist H. V. Collet-Descotils, who actually published his paper one month before Tennant, but
Tennant is given credit for the discovery, perhaps because he alone also found osmium in the ore.


