Lanthanum
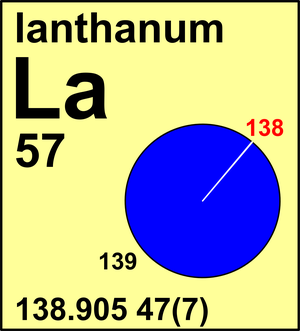
| Isotope | Atomic mass (Da) | Isotopic abundance (amount fraction) |
|---|---|---|
| 138La | 137.907 12(2) | 0.000 8881(71) |
| 139La | 138.906 36(2) | 0.999 1119(71) |
In 1961, the Commission recommended a value of Ar(La) = 138.91, based on the recalculation of chemical determinations and mass-spectrometric determinations. In 1969, the Commission recommended Ar(La) = 138.9055(3) based upon the same determinations, taking into account that two additional digits were justified in view of the small influence any mass discrimination could have on the atomic weight of this nearly monoisotopic element. In effect, the chemical determinations were no longer considered significant. In 1985, the Commission re-examined the available data and decided to revise Ar(La) to 138.9055(2), and then to 138.905 47(7) in 2005.
The minor isotope 138La is radioactive with a half-life of 1.06(4)×1011 a, with 138Ba and 138Ce as decay products. The atomic weight of all three elements remains unaffected even over geologic time periods. The "g" annotation arises from the presence of naturally occurring fission products found in fossil reactors at Gabon, south-west Africa.
© IUPAC 2003
CIAAW
Lanthanum
Ar(La) = 138.905 47(7) since 2005
The name derives from the Greek lanthanein for "to be hidden" or "to escape notice" because it hid in
cerium ore and was difficult to separate from that rare earth mineral. Lanthanum was discovered by the Swedish
surgeon and chemist Carl-Gustav Mosander in 1839. In 1842, Mosander separated his lanthanium sample
into two oxides; for one of these he retained the name lanthanum and for the other he gave the name
didymium (or twin).


