Lead
| Isotope | Atomic mass (Da) | Isotopic abundance (amount fraction) |
|---|---|---|
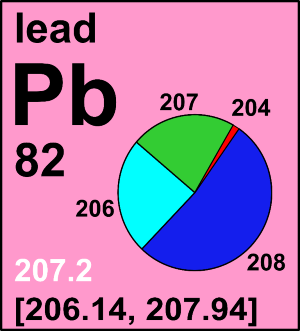
| 204Pb | 203.973 043(8) | [0.0000, 0.0158] |
| 206Pb | 205.974 465(8) | [0.0190, 0.8673] |
| 207Pb | 206.975 897(8) | [0.0035, 0.2351] |
| 208Pb | 207.976 652(8) | [0.0338, 0.9775] |
The atomic weight of lead is quite variable in nature because the three heaviest isotopes are the stable end-products of the radioactive decay of uranium (238U to 206Pb and 235U to 207Pb) and thorium (232Th to 208Pb). These variations in isotope ratios and atomic weights provide useful information in many areas of science, including geochronology, archaeology, environmental studies, and forensic science. While elemental lead can serve as an abundant and homogeneous isotopic reference, deviations from the isotope ratios in other lead occurrences limit the accuracy with which a standard atomic weight can be given for lead.
Recognizing this, in 1961, the Commission recommended an atomic weight of 207.19 that was based on the chemical measurements, and stated that "...it quite well represented the lead most likely to be encountered in normal laboratory work". However, the Commission's policy now aims for the implied range of the standard atomic weights to cover all normal sources of an element. In the 1969 report, the Commission considered natural variations in the atomic weight of lead ranging from 207.184 to 207.293 and recommended the value of Ar(Pb) = 207.2(1).
In a comprehensive review of several hundred publications and analyses of more than 8000 samples (Zhu et al, 2021), published isotope data indicate that the lowest reported lead atomic weight of a normal terrestrial material is 206.1462 ± 0.0028 (k = 2), determined for a growth of the phosphate mineral monazite from the Lewisian complex in north-western Scotland, which contains mostly 206Pb and almost no 204Pb. The highest published lead atomic weight 207.9351 ± 0.0005 (k = 2) is for monazite from a micro-inclusion. The material is also from the Lewisian complex in north-western Scotland containing almost pure radiogenic 208Pb. Assigning the aforementioned lead atomic weights as the lower and upper bounds of the interval, the standard atomic weight of lead is [206.14, 207.94]. If a single atomic-weight value is needed, the Commission recommends using 207.2 ± 1.1, which corresponds to the common lead with a symmetric uncertainty covering normal materials.
The large variations in isotope ratios and atomic weights of lead in normal materials mean that reliable measurements of lead should employ laboratory standards with known isotopic composition of lead. As an example, the nominal isotopic composition of lead in NIST SRM 981 reference material has an atomic weight of 207.2 with isotopic composition x(204Pb) = 0.014 mol/mol, x(206Pb) = 0.241 mol/mol, x(207Pb) = 0.221 mol/mol, and x(208Pb) = 0.524 mol/mol.
The decay of uranium and thorium to lead permits geological age determinations to be made of minerals containing the heavy radioactive elements. Extensive use of lead over the history of mankind has led to widespread pollution, and the isotope-abundance variations reflected in the atomic weights enable historical and modern sources to be identified.
© IUPAC 2021
CIAAW
Lead
Ar(Pb) = [206.14, 207.94] since 2020
The name derives from the Anglo-Saxon lead, which is of unknown origin. The element was known
from prehistoric times. The chemical symbol Pb is derived from the Latin plumbum.
Isotopic reference materials of lead.
For more information about the natural variations of the atomic weight of lead please read IUPAC Technical Report Variation of lead isotopic composition and atomic weight in terrestrial materials (IUPAC Technical Report)  by Z.-K. Zhu et al Pure Appl. Chem. 93, 155-166 (2021).
by Z.-K. Zhu et al Pure Appl. Chem. 93, 155-166 (2021).


