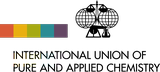Lithium
| Isotope | Atomic mass (Da) | Isotopic abundance (amount fraction) |
|---|---|---|
| 6Li | 6.015 122 89(1) | [0.019, 0.078] |
| 7Li | 7.016 003 44(3) | [0.922, 0.981] |
Although Li occurs in diverse geological associations and although the relative mass difference of the isotopes is large, the variability of the atomic-weight values of lithium in most terrestrial sources appears to be smaller than 0.002. The lowest 7Li abundance reported for a naturally occurring sample is from dissolved lithium in groundwater from a coastal aquifer in South Carolina with x(7Li) = 0.9227 and Ar(Li) = 6.9387. The highest 7Li abundance reported in a naturally occurring sample is from lithium in pore water from a marine sediment core with x(7Li) = 0.9278 and Ar(Li) = 6.9438.
The minor isotope 6Li is a potentially valuable nuclear source material for tritium production, an important component in hydrogen bombs, and a neutron absorber for the nuclear-fusion reaction. Lithium depleted in 6Li may be distributed in commerce, with abundances of 6Li as low as 2 % and atomic weights in excess of 6.99. This is the justification for the "m" annotation. In 1993, the Commission expressed concern about the availability on the commercial market of such depleted materials and decided to put the atomic-weight value and uncertainty between square brackets and to add a dagger symbol to warn that, if a more accurate value is required, it must be determined on a sample of the material concerned.
In 1995, the Commission recommended that all δ(7Li) values be reported relative to the lithium carbonate reference material LSVEC.
Atomic weights of the elements 2009 by M.E. Wieser and T.B. Coplen. Pure Appl. Chem. 2011 (83) 359-396
CIAAW
Lithium
Ar(Li) = [6.938, 6.997] since 2009
The name derives from the Latin lithos for "stone" because lithium was thought to exist only in minerals
at that time. It was discovered by the Swedish mineralogist Johan August Arfwedson in 1818 in the
mineral petalite LiAl(Si2O5)2. Lithium was isolated in 1855 by the German chemists Robert Wilhelm Bunsen
and Augustus Matthiessen.
Natural variations of lithium isotopic composition
Isotopic reference materials of lithium.