Lutetium
| Isotope | Atomic mass (Da) | Isotopic abundance (amount fraction) |
|---|---|---|
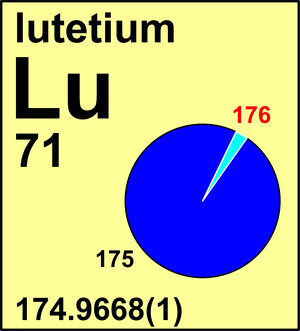
| 175Lu | 174.940 777(8) | 0.974 14(5) |
| 176Lu | 175.942 692(8) | 0.025 86(5) |
In 1961, the Commission recommended Ar(Lu) = 174.97 based on new mass-spectrometric determinations. The Commission also noted the close agreement with the chemical determinations Ar(Lu). In 1977, it took note of a new isotope-abundance determination, which was deemed more accurate but, like its predecessors, was not calibrated. Nevertheless, as a result, the more precise Ar(Lu) = 174.967(3) was recommended. Since then, the Commission was able to reduce the quoted uncertainty based on new mass spectrometric measurements that agreed with earlier determinations. The Commission last revised the atomic weight of lutetium in 2024 based on recent multi-collector ICPMS measurements of its isotopic composition.
The minor isotope, 176Lu, is radioactive with a half-life of 3.57(14)×1010 a and the 176Lu–176Hf decay system is used as a geochronometer. In consequence, Ar(Lu) will change comparably with the current uncertainty (0.000 05) in only about 1×108 years. At the Oklo fossil natural nuclear reactors at Gabon, south-west Africa, the n(176Lu)/n(175Lu) ratio has been used as a sensitive measure of the average equilibrium temperature of the neutrons at the time of the nuclear reactions. The occurrence, at this site, of almost pure (> 99.4 %) isotope 175Lu justifies the annotation "g" to the standard atomic weight of lutetium indicating that geological materials are known in which the element has an isotopic composition outside the limits for normal material.
CIAAW
Lutetium
Ar(Lu) = 174.966 69 ± 0.000 05 since 2024
The name derives from Lutetia, the ancient name for the city of Paris. The discovery of lutetium is credited to the
French chemist Georges Urbain in 1907 although it had been separated earlier and independently by the
Austrian chemist Carl Auer (Baron von Welsbach) from an ytterbium sample.
Von Welsbach had named the element
cassiopeium after the constellation Cassiopeia. However, because Urbain published his results
before Auer, his name for the element was adopted by IUPAC in 1949.


