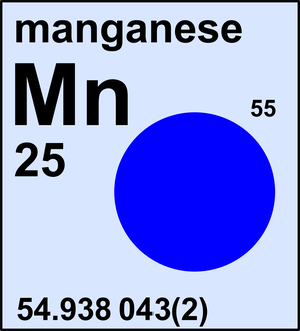
Manganese
| Isotope | Atomic mass (Da) | Isotopic abundance (amount fraction) |
|---|---|---|
| 55Mn | 54.938 043(2) | 1 |
Manganese is a monoisotopic element and its atomic weight is determined solely by its isotope 55Mn. The Commission last revised the standard atomic weight of manganese in 2017 based on the latest Atomic Mass Evaluation by IUPAP.
53Mn is radioactive with a half-life of 3.7(2) Ma, too short for survival of a detectable amount of primordial isotope. However, 53Mn has been identified on earth as a cosmic-ray product and as a constituent of cosmic dust amounting to approx. one disintegration per minute per gram of manganese in sediment cores corresponding to an abundance of 3x10-13, much too small to affect the standard atomic weight.
© IUPAC 2003
CIAAW
Manganese
Ar(Mn) = 54.938 043(2) since 2017
The name derives from the Latin magnes for "magnet" since pyrolusite (MnO2) has magnetic properties.
It was discovered by the Swedish pharmacist and chemist Carl-Wilhelm Scheele in 1774. In the same year,
the Swedish chemist Johan Gottlieb Gahn first isolated the metal.


