Neodymium
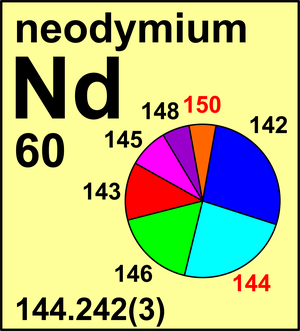
| Isotope | Atomic mass (Da) | Isotopic abundance (amount fraction) |
|---|---|---|
| 142Nd | 141.907 73(1) | 0.271 52(40) |
| 143Nd | 142.909 82(1) | 0.121 74(26) |
| 144Nd | 143.910 09(1) | 0.237 98(19) |
| 145Nd | 144.912 58(1) | 0.082 93(12) |
| 146Nd | 145.913 12(1) | 0.171 89(32) |
| 148Nd | 147.916 90(2) | 0.057 56(21) |
| 150Nd | 149.920 902(9) | 0.056 38(28) |
Two isotopes of neodymium, 144Nd and 145Nd, are radioactive but with half-lives so long (2.1(4)×1015 a, and more than 6×1016 a, respectively) that there is no measurable effect on the atomic weight of Nd comparable with the precision of the standard atomic weight. 143Nd is the decay product of radioactive 147Sm.
Although the resulting fluctuations of n(143Nd)/n(144Nd) are also too small to affect Ar(Nd), they are measurable and do permit deductions to be made in geochronology and in geochemical phenomena, for instance, the mixing of ocean currents. For such applications, precise measurements of isotope abundances and Sm/Nd ratios are needed, and these are greatly facilitated by comparisons with standard solutions. The "g" notation arises from the presence of naturally occurring fission products found in fossil reactors at Gabon, south-west Africa.
© IUPAC 2003
CIAAW
Neodymium
Ar(Nd) = 144.242(3) since 2005
The name derives from the Greek neos for "new" and didymos for "twin". It was discovered by the
Swedish surgeon and chemist Carl Gustav Mosander in 1841, who called it didymium (or twin) because
of its similarity to lanthanum, which he had previously discovered two years earlier. In 1885, the
Austrian chemist Carl Auer (Baron von Welsbach) separated didymium into two elements, one of which he
called neodymium (or new twin).


