Nickel
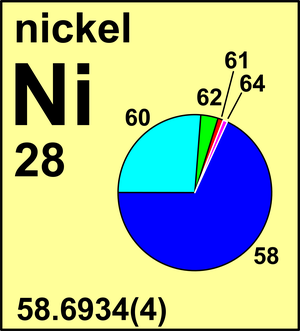
| Isotope | Atomic mass (Da) | Isotopic abundance (amount fraction) |
|---|---|---|
| 58Ni | 57.935 342(3) | 0.680 769(190) |
| 60Ni | 59.930 785(3) | 0.262 231(150) |
| 61Ni | 60.931 055(3) | 0.011 399(13) |
| 62Ni | 61.928 345(3) | 0.036 345(40) |
| 64Ni | 63.927 966(3) | 0.009 256(19) |
All the chemical and mass-spectrometric measurements have agreed to establish a value of Ar(Ni) = 58.70(1). Nevertheless, that value is a little higher than the average (58.694) of Baxter's excellent determinations, whose credibility is increased by the proof of accuracy of the parallel work on the atomic weight of cobalt.
At its 1989 meeting, the Commission changed its recommended value for the atomic weight of Ni to Ar(Ni) = 58.6934(2) based on recent mass-spectrometric determinations. The excellent agreement between the average of the chemical values and this new determination illustrates the remarkable accuracy of the determinations of Baxter and associates. In addition, a comparison of the isotopic composition of nickel in many minerals, salts, and metals showed no statistically significant variations. Therefore, no additional allowance to the overall uncertainty was necessary because the isotopic composition of terrestrial Ni is apparently invariant within the measurement uncertainty. The Commission increased the uncertainty of the standard atomic weight of nickel in 2007.
It should be noted that the atomic weight of nickel is now one of the most accurately known for a polyisotopic element.
© IUPAC 2003
CIAAW
Nickel
Ar(Ni) = 58.6934(4) since 2007
The name derives from the German Nickel for "deceptive little spirit" because miners called mineral niccolite
(NiAs) by the name Kupfernickel (false copper) because it resembled copper ores in appearance,
but no copper was found in the ore. It was discovered by the Swedish metallurgist Axel-Frederik Cronstedt in 1751.
Isotopic reference materials of nickel.


