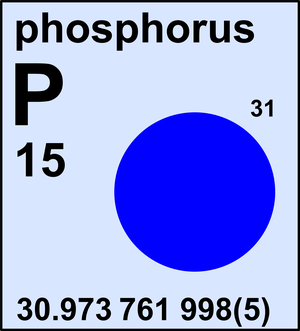
Phosphorus
| Isotope | Atomic mass (Da) | Isotopic abundance (amount fraction) |
|---|---|---|
| 31P | 30.973 761 998(5) | 1 |
Phosphorus is a monoisotopic element and its atomic weight is determined solely by its isotope 31P. The Commission last revised the standard atomic weight of phosphorus in 2013 based on the latest Atomic Mass Evaluation by IUPAP.
32P and 33P are cosmogenic and also can be produced by the nuclear industry, but their concentrations in normal materials are too small to affect Ar(P).
© IUPAC 2003
CIAAW
Phosphorus
Ar(P) = 30.973 761 998(5) since 2013
The name derives from the Greek phosphoros for "bringing light" because it has the property of glowing
in the dark. This was also the ancient name for the planet Venus, when it appears before sunrise.
Phosphorus was discovered by the German merchant Hennig Brand in 1669.


