Potassium
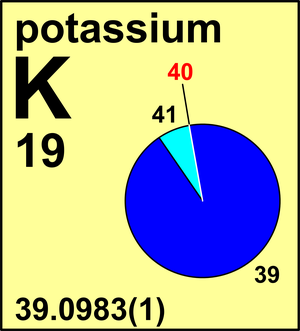
| Isotope | Atomic mass (Da) | Isotopic abundance (amount fraction) |
|---|---|---|
| 39K | 38.963 706 49(3) | 0.932 581(44) |
| 40K | 39.963 9982(4) | 0.000 117(1) |
| 41K | 40.961 825 26(3) | 0.067 302(44) |
In 1975, the Commission recommended Ar(K) = 39.0983(3), based on new mass-spectrometric measurements and results of a mineralogical study of possible isotopic variations. This uncertainty was reduced to 0.0001 in 1979, based on an evaluation of possible variations of the isotope abundances and the effects of small errors in the abundance measurements.
The Commission added the annotation "g" in 1991 because of the reports indicating values outside the atomic-weight uncertainty. However, this annotation was removed in 1995 when these claims could not be supported.
The minor isotope, 40K, is radioactive with a total half-life of 1.26(1) Ga, and decays to both 40Ar and 40Ca. As a result of this decay, Ar(K) will decrease by approximately 1.5 parts in million in one half-life. The K/Ar and K/Ca decay systems are used extensively in geochronology.
© IUPAC 2003
CIAAW
Potassium
Ar(K) = 39.0983(1) since 1979
The name derives from the English "potash" or "pot ashes" because it is found in caustic potash (KOH).
The symbol K derives from the Latin kalium via the Arabic qali for alkali. It was first isolated
by the British chemist Humphry Davy in 1807 from electrolysis of potash (KOH).


