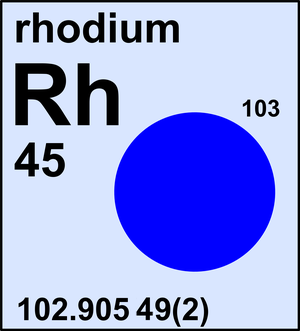
Rhodium
| Isotope | Atomic mass (Da) | Isotopic abundance (amount fraction) |
|---|---|---|
| 103Rh | 102.905 49(2) | 1 |
Rhodium is a monoisotopic element and its atomic weight is determined solely by its isotope 103Rh. The Commission last revised the standard atomic weight of rhodium in 2017 based on the latest Atomic Mass Evaluation by IUPAP.
© IUPAC 2003
CIAAW
Rhodium
Ar(Rh) = 102.905 49(2) since 2017
The name derives from the Greek rhodon for rose because of the rose color of dilute solutions of its
salts. It was discovered by the English chemist and physicist William Hyde Wollaston in 1803 in a crude platinum ore.


