Samarium
| Isotope | Atomic mass (Da) | Isotopic abundance (amount fraction) |
|---|---|---|
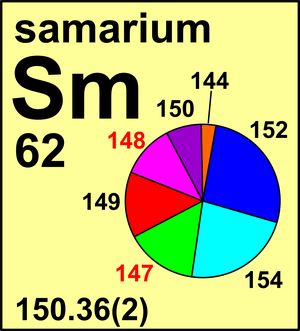
| 144Sm | 143.912 01(1) | 0.0308(4) |
| 147Sm | 146.914 90(1) | 0.1500(14) |
| 148Sm | 147.914 83(1) | 0.1125(9) |
| 149Sm | 148.917 191(9) | 0.1382(10) |
| 150Sm | 149.917 282(9) | 0.0737(9) |
| 152Sm | 151.919 739(8) | 0.2674(9) |
| 154Sm | 153.922 22(1) | 0.2274(14) |
In its 1961 report, the Commission expressed concern about disparities among several isotope-abundance measurements for samarium, while chemical measurements yielded persistently higher values. Thus, the Commission retained the 1955 value of Ar(Sm) = 150.35. In 1969, the Commission resolved that the evidence for the current atomic weight is not sufficient and recommended an atomic weight with large uncertainty, Ar(Sm) = 150.4(1), so that the new value included almost all chemical and mass-spectrometric data.
In 1979, the Commission undertook a thorough review of the literature and the chemical determinations by Hönigschmid and Hirschbold-Wittner were found most reliable because the purity of SmCl3 was established by x-ray fluorescence and optical-emission spectrometry. Their data suggested Ar(Sm) = 150.36. Additionally, in 1975, precise mass-spectrometric measurements gave Ar(Sm) = 150.366. As a result, in 1979, the Commission recommended Ar(Sm) = 150.36(3), a vindication of the 1961 value. This value has remained unchanged until 2005 when it was slightly revised.
Isotopes 147Sm and 148Sm have very long half-lives of 1.06(1)×1011 a and 7(3)×1015 a, respectively. They cannot appreciably influence Ar(Sm) even over geologic time intervals. The "g" notation arises from the presence of naturally occurring fission products found in fossil reactors at Gabon, south-west Africa.
© IUPAC 2003
CIAAW
Samarium
Ar(Sm) = 150.36(2) since 2005
The name derives from the mineral samarskite, in which it was found and that had been named for
Colonel Samarski, a Russian mine official. Samarium was originally discovered in 1878 by the Swiss chemist
Marc Delafontaine, who called it decipium. It was also discovered by the French chemist Paul-Emile
Lecoq de Boisbaudran in 1879. In 1881, Delafontaine determined that his decipium could be resolved
into two elements, one of which was identical to Boisbaudran's samarium. In 1901, the French chemist
Eugène-Anatole Demarçay showed that this samarium earth also contained europium.