Selenium
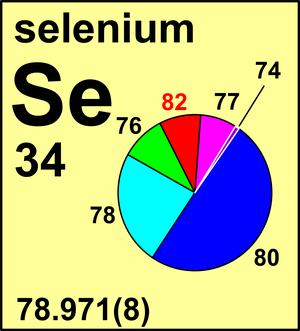
| Isotope | Atomic mass (Da) | Isotopic abundance (amount fraction) |
|---|---|---|
| 74Se | 73.922 4759(1) | 0.0086(3) |
| 76Se | 75.919 2137(1) | 0.0923(7) |
| 77Se | 76.919 9141(5) | 0.0760(7) |
| 78Se | 77.917 309(1) | 0.2369(22) |
| 80Se | 79.916 522(6) | 0.4980(36) |
| 82Se | 81.916 699(3) | 0.0882(15) |
In 1934, the Commission recommended Ar(Se) = 78.96 based on the chemical determinations. This value was reaffirmed in 1969 when Ar(Se) = 78.96(3) was recommended. Both the atomic weight and the uncertainty values have since remained unchanged until 2013 when new mass-spectrometric measurements enabled to recommend a more precise value Ar(Se) = 78.971(8).
82Se is radioactive with an enormously long half-life of approximately 1020 a and is considered to be a stable isotope.
© IUPAC 2003
CIAAW
Selenium
Ar(Se) = 78.971(8) since 2013
The name derives from the Greek Selene, who was the Greek goddess of the Moon because the element
is chemically found with tellurium (Tellus was the Roman goddess of the Earth). Selenium was discovered by the
Swedish chemist Jöns Jacob Berzelius in 1817, while trying to isolate tellurium in an impure sample.
Isotopic reference materials of selenium.


