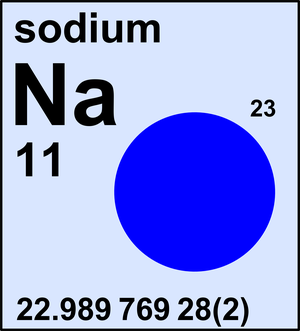
Sodium
| Isotope | Atomic mass (Da) | Isotopic abundance (amount fraction) |
|---|---|---|
| 23Na | 22.989 769 28(2) | 1 |
Sodium is a monoisotopic element and its atomic weight is determined solely by its isotope 23Na. The Commission last revised the standard atomic weight of sodium in 2005 based on the latest Atomic Mass Evaluation by IUPAP.
© IUPAC 2003
CIAAW
Sodium
Ar(Na) = 22.989 769 28(2) since 2005
The name derives from the English soda and Latin sodanum for "headache remedy". The symbol Na derives from the Latin natrium for "natron" (soda in English). Sodium was discovered in 1807 by the
English chemist Humphry Davy from electrolysis of caustic soda (NaOH).