Tellurium
| Isotope | Atomic mass (Da) | Isotopic abundance (amount fraction) |
|---|---|---|
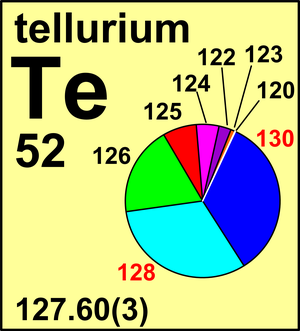
| 120Te | 119.904 06(2) | 0.0009(1) |
| 122Te | 121.903 04(1) | 0.0255(12) |
| 123Te | 122.904 27(1) | 0.0089(3) |
| 124Te | 123.902 82(1) | 0.0474(14) |
| 125Te | 124.904 43(1) | 0.0707(15) |
| 126Te | 125.903 31(1) | 0.1884(25) |
| 128Te | 127.904 461(6) | 0.3174(8) |
| 130Te | 129.906 222 75(8) | 0.3408(62) |
In 1961, the Commission adopted Ar(Te) = 127.60 based on the chemical-ratio determinations. In the absence of calibrated mass-spectrometric measurements, the value of Ar(Te) = 127.60(3), adopted in 1969, has been retained as the standard atomic weight of tellurium.
123Te, 128Te, and 130Te are radioactive; the minor isotope 123Te has a long half-life of 1.3(4)×1013 a and transforms into 123Sb without significantly affecting either element's atomic weight even over geological time. The major isotopes 128Te and 130Te have long half-lives of approximately 1024 a and 1021 a, respectively. These isotopes suffer double β– decay and are responsible for detectable xenon isotopic anomalies in old Te-bearing minerals.
The "g" annotation for Te arises from the presence of naturally occurring fission products found in fossil reactors at Gabon, south-west Africa.
© IUPAC 2003
CIAAW
Tellurium
Ar(Te) = 127.60(3) since 1969
The name derives from the Latin Tellus, who was the Roman goddess of the Earth. Tellurium was discovered by
Franz Joseph Müller von Reichenstein in 1782 and overlooked for 15
years until it was isolated by the German chemist Martin-Heinrich Klaproth in 1798. The Hungarian
chemist Paul Kitaibel independently discovered tellurium in 1789, prior to Klaproth's work but after
von Reichenstein.


