Thallium
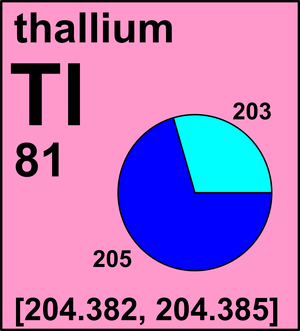
| Isotope | Atomic mass (Da) | Isotopic abundance (amount fraction) |
|---|---|---|
| 203Tl | 202.972 344(8) | [0.2944, 0.2959] |
| 205Tl | 204.974 427(8) | [0.7041, 0.7056] |
In 1961, the Commission recommended Ar(Tl) = 204.37 based on the chemical determinations. In 1979, the Commission considered new mass-spectrometric measurement and recommended Ar(Tl) = 204.383(1). Recent studies have indicated substantial variation in n(205Tl)/n(203Tl) of natural materials, which led the Commission to adopt the interval notation for the standard atomic weight of thallium in 2009.
Atomic weights of the elements 2009 by M.E. Wieser and T.B. Coplen. Pure Appl. Chem. 2011 (83) 359-396
CIAAW
Thallium
Ar(Tl) = [204.382, 204.385] since 2009
The name derives from the Greek thallos for "green shoot" or "twig" because of the bright green line in
its spectrum. Thallium was discovered by the English physicist and chemist William Crookes in 1861.
Metallic thallium was first isolated by the French chemist Claude-Auguste Lamy in 1862.
Natural variations of thallium isotopic composition
Isotopic reference materials of thallium.


