Uranium
| Isotope | Atomic mass (Da) | Isotopic abundance (amount fraction) |
|---|---|---|
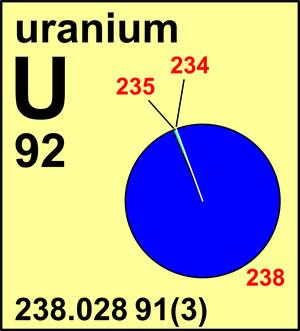
| 234U | 234.040 950(8) | 0.000 054(5) |
| 235U | 235.043 928(8) | 0.007 204(6) |
| 238U | 238.050 79(1) | 0.992 742(10) |
In 1969, the Commission recommended Ar(U) = 238.029(1) for the atomic weight of U based on mass-spectrometric determinations and a careful analysis of the variability of x(235U) in nature. In 1979, the Commission took note of later studies of the variations of the 235U abundance in normal sources, which justified a more precise value for the standard atomic weight, thus leading to Ar(U) = 238.0289(1). The atomic weight and uncertainty of uranium were changed to 238.028 91(3) in 1999 on the basis of new calibrated mass-spectrometric measurements.
That value applies to uranium as found in normal terrestrial sources, except as discovered in one locality in south-west Africa (Gabon at Oklo), hence the annotation "g". Uranium is used in the nuclear fuel cycle either enriched or depleted in 235U, hence the annotation "m".
All uranium isotopes are α-emitters. Isotopes 235U and 238U are primordial with the 235U abundance declining very gradually in geological time because of its faster decay. 234U, itself a decay product of 238U, is in equilibrium established by the ratio of the half-lives. 235U decays by a branched series ending with 207Pb, 238U (and 234U) by a similar series ending in 206Pb. The 238U–206Pb and the 235U–207Pb decay systems are of fundamental importance in geochronology.
© IUPAC 2003
CIAAW
Uranium
Ar(U) = 238.028 91(3) since 1999
The name derives from the planet Uranus, which in Roman mythology was "Father Heaven". The
German chemist Martin-Heinrich Klaproth discovered the element in 1789, following William Hershel's discovery of the planet in 1781.
The metallic uranium was first isolated by the French chemist Eugène-Melchior Peligot in 1841.
Isotopic reference materials of uranium.


