Zinc
| Isotope | Atomic mass (Da) | Isotopic abundance (amount fraction) |
|---|---|---|
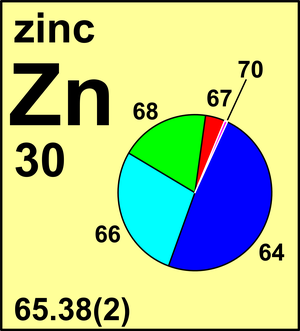
| 64Zn | 63.929 142(5) | 0.4917(75) |
| 66Zn | 65.926 034(5) | 0.2773(98) |
| 67Zn | 66.927 127(5) | 0.0404(16) |
| 68Zn | 67.924 844(5) | 0.1845(63) |
| 70Zn | 69.925 32(2) | 0.0061(10) |
In 1961, the Commission recommended Ar(Zn) = 65.37 based on the chemical determinations. Meanwhile, mass-spectrometric determinations yielded a higher value of Ar(Zn) = 65.387.
In 1971, coulometric determinations yielded Ar(Zn) = 65.377(3), whereupon the Commission changed the recommended value to 65.38(1). Soon, another mass-spectrometric value was published which yielded Ar(Zn) = 65.396(5). Faced with this ongoing discrepancy between chemical and physical values, in 1983 the Commission recommended Ar(Zn) = 65.39(2), explaining that the value was now weighted toward the mass-spectrometric measurement, but the uncertainty included the coulometric measurement. In 2001, Ar(Zn) was changed to 65.409(4) and in 2007 the Commission acknowledged that data influencing the 2001 decision could no longer be supported and recommended the current value of Ar(Zn) = 65.38(2). This change was unique in the sense that for the first time in the history of the Commission, the standard atomic weight (with its uncertainty) was outside the bounds of the previous value.
CIAAW
Zinc
Ar(Zn) = 65.38(2) since 2007
The name derives from the German zink of unknown origin. It was first used in prehistoric times, where
its compounds were used for healing wounds and sore eyes and for making brass. Zinc was recognized as
a metal as early as 1374.
Isotopic reference materials of zinc.


