Zirconium
| Isotope | Atomic mass (Da) | Isotopic abundance (amount fraction) |
|---|---|---|
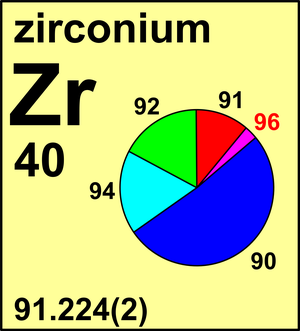
| 90Zr | 89.904 6988(8) | 0.5147(6) |
| 91Zr | 90.905 6402(7) | 0.1123(6) |
| 92Zr | 91.905 0353(7) | 0.1716(4) |
| 94Zr | 93.906 313(1) | 0.1736(7) |
| 96Zr | 95.908 2776(8) | 0.0278(4) |
The atomic weight of Zr has been taken to be Ar(Zr) = 91.22 since 1931, and this value was reconfirmed in 1969 when it was assessed as 91.22(1). Since then, several isotopic composition measurements have been carried out, including recent measurements by TIMS and MC-ICPMS which prompted a revision of its standard atomic weight in 2024.
CIAAW
Zirconium
Ar(Zr) = 91.222 ± 0.003 since 2024
The name derives from the Arabic zargun for "gold-like". It was discovered in zirconia by the German
chemist Martin-Heinrich Klaproth in 1789. Zirconium was first isolated by Swedish chemist Jöns Jacob
Berzelius in 1824 in an impure state, and finally by the chemists D. Lely, Jr. and L. Hamburger in a pure
state in 1914.


