Boron
| Isotope | Atomic mass (Da) | Isotopic abundance (amount fraction) |
|---|---|---|
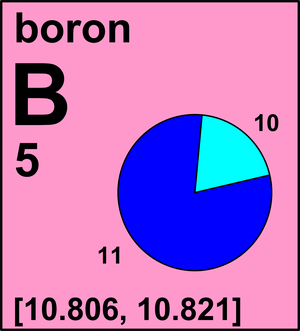
| 10B | 10.012 9369(1) | [0.189, 0.204] |
| 11B | 11.009 305 17(8) | [0.796, 0.811] |
In 1961, the Commission recommended Ar(B) = 10.811(3) based on calibrated mass-spectrometric measurements on brines and minerals from Searles Lake. The uncertainty was based on reported variations in natural abundances. In 1981, the Commission concluded that the range of isotope-abundance variations typical of the most common sources is covered by Ar(B) = 10.811(2). This value includes major commercial sources of boron in California and Turkey. However, the existence of normal terrestrial occurrences with sample atomic weights outside these implied limits could not be denied. Under the 1983 policy of a more liberal use of any single-digit uncertainty, the Commission changed the recommended standard atomic weight to Ar(B) = 10.811(5). In 1985, the "g" annotation was added to reflect the occurrence of materials with anomalous isotopic compositions. Compared with most other standard atomic weights, the tabulated value for boron is relatively uncertain. That uncertainty increased in 1995 when the Commission decided to include the isotopic composition of boron in seawater.
Natural variations in n(11B)/n(10B) are reported as δ(11B) values relative to the reference material NIST SRM 951a, which has n(11B)/n(10B) = 4.0437(33). Reported δ951a(11B) values range from a low of −34.2 ‰ (x(11B) = 0.7961 and Ar(B) = 10.8062) in a metamorphic mineral sample from Antarctica to a high of +59.2 ‰ (x(11B) = 0.8107 and Ar(B) = 10.8207) in brine from a volcanic crater lake in south-eastern Australia. Separated and enriched isotopes of boron are commercially available; the atomic weights of such samples can differ from one another by up to almost 10 %. Although the Commission is unaware of undisclosed commercial sales of such material, an "m" annotation warns users of the possibility of its inadvertent dissemination.
Atomic weights of the elements 2009 by M.E. Wieser and T.B. Coplen. Pure Appl. Chem. 2011 (83) 359-396
CIAAW
Boron
Ar(B) = [10.806, 10.821] since 2009
The name derives from the Arabic buraq for "white". Although its compounds were known for thousands
of years, it was not isolated until 1808 by the French chemists Louis-Joseph Gay-Lussac and Louis-Jacques Thenard.
Natural variations of boron isotopic composition
Isotopic reference materials of boron.


