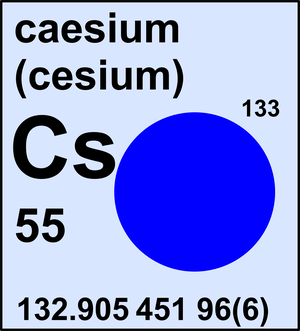
Caesium
| Isotope | Atomic mass (Da) | Isotopic abundance (amount fraction) |
|---|---|---|
| 133Cs | 132.905 451 96(6) | 1 |
Caesium is a monoisotopic element and its atomic weight is determined solely by its isotope 133Cs. The Commission last revised the standard atomic weight of caesium in 2013 based on the latest Atomic Mass Evaluation by IUPAP.
© IUPAC 2003
CIAAW
Caesium
Ar(Cs) = 132.905 451 96(6) since 2013
The name derives from the Latin caesius for "sky blue", which was the colour of the caesium line
in the spectroscope. Caesium was discovered by the German chemist Robert Wilhelm Bunsen and the German
physicist Gustav Robert Kirchhoff in 1860. It was first isolated by the German chemist Carl Setterberg
in 1882.


