Chromium
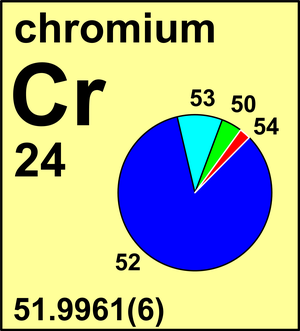
| Isotope | Atomic mass (Da) | Isotopic abundance (amount fraction) |
|---|---|---|
| 50Cr | 49.946 041(3) | 0.043 45(13) |
| 52Cr | 51.940 505(3) | 0.837 89(18) |
| 53Cr | 52.940 647(3) | 0.095 01(17) |
| 54Cr | 53.938 878(3) | 0.023 65(7) |
In 1983 the Commission recommended the standard atomic weight of chromium to four decimal places, Ar(Cr) = 51.9961(6). Meanwhile, report isotope fractionation of chromium during chromate reduction, resulting in δ53CrSRM979 values in groundwater samples as high as +5.8 ‰ or Ar(Cr) = 51.9982, which is outside the current range of uncertainty of the standard atomic weight. Measurements of n(53Cr)/n(52Cr) can be expressed as δ53Cr values with respect to NIST SRM 979.
© IUPAC 2003
CIAAW
Chromium
Ar(Cr) = 51.9961(6) since 1983
The name derives from the Greek chroma for "colour", from the many coloured compounds of chromium.
It was discovered in 1797 by the French chemist and pharmacist Nicolas-Louis Vauquelin, who also isolated
chromium in 1798.
Isotopic reference materials of chromium.


