Gadolinium
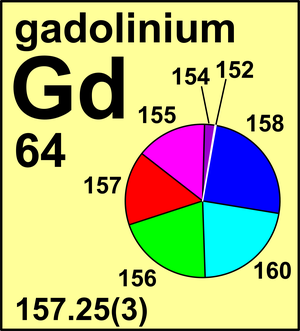
| Isotope | Atomic mass (Da) | Isotopic abundance (amount fraction) |
|---|---|---|
| 152Gd | 151.919 799(8) | 0.002 04(2) |
| 154Gd | 153.920 873(8) | 0.021 87(9) |
| 155Gd | 154.922 630(8) | 0.148 28(60) |
| 156Gd | 155.922 131(8) | 0.204 93(22) |
| 157Gd | 156.923 968(8) | 0.156 57(17) |
| 158Gd | 157.924 112(8) | 0.248 20(20) |
| 160Gd | 159.927 062(9) | 0.218 11(28) |
In 1969, the Commission recommended the atomic weight of gadolinium to be Ar(Gd) = 157.25(3) based on mass-spectrometric determinations made in the 1940s. It has remained unchanged until 2024 when two independent studies dedicated to the measurement of isotopic composition of gadolinium prompted a revision of its standard atomic weight.
152Gd has a very long half-life in excess of 1014 a. Within the life time of the earth, this radioactivity will not have affected the atomic weight to the precision here quoted. Some rare terrestrial materials can have abnormal isotopic compositions of gadolinium with extreme isotopic composition being a sample from the vicinity of the Oklo fossil natural nuclear reactors at Gabon, south-west Africa, having an atomic-weight value of 156.57.
CIAAW
Gadolinium
Ar(Gd) = 157.249 ± 0.002 since 2024
The name derives from the mineral gadolinite, in which it was found, and that had been named for the
Finnish rare earth chemist Johan Gadolin. Gadolinium was discovered by the Swiss chemist Jean-Charles
Galissard de Marignac in 1886, who produced a white oxide in a samarskite mineral. In
1886, the French chemist Paul-Emile Lecoq de Boisbaudran gave the name gadolinium.


