Nitrogen
| Isotope | Atomic mass (Da) | Isotopic abundance (amount fraction) |
|---|---|---|
| 14N | 14.003 074 004(2) | [0.995 78, 0.996 63] |
| 15N | 15.000 108 899(4) | [0.003 37, 0.004 22] |
The primary reference material for the relative abundance measurements of nitrogen isotopes is atmospheric N2, which is homogeneous with respect to analytical uncertainties and is assigned a δair(15N) value of 0 ‰. Relative isotope-ratio measurements of nitrogen commonly have uncertainties of the order of 0.1 ‰, which is significantly smaller than the reported uncertainty of the calibrated "best measurement" (1.1 ‰). Variations in the isotopic composition of nitrogen in chemical reagents and natural terrestrial systems are known to exceed 200 ‰, which is much larger than the uncertainty due to either relative or "absolute" isotope-ratio measurements. Therefore, the accuracy and precision of the standard atomic weight of nitrogen are limited almost entirely by real variations, hence the annotation "r".
Measurable variations in the isotope abundances (and atomic weights) of nitrogen are found in most nitrogen compounds. The vast majority of chemical reagents, manufactured fertilizers, and environmental samples have δ(15N) values between about −15 and +20 ‰ which corresponds to x(15N) = 0.003 61 to 0.003 74 and Ar(N) = 14.006 67 to 14.006 80. Isotope fractionations are caused by physical, chemical, and biological processes. Some of the largest common effects in the natural environment are caused by microbially mediated oxidation and reduction reactions and by ammonia or nitric acid evaporation.
The most 15N-enriched occurrences reported in nature include dissolved nitrate that had undergone partial microbial reduction (denitrification) in groundwater (e.g., δ(15N) = +103 ‰, x(15N) = 0.004 039, and Ar(N) = 14.007 10), and nitrate in Antarctic ice that may have been fractionated by evaporation of HNO3 with δ(15N) = +150 ‰, x(15N) = 0.004 210, and Ar(N) = 14.007 27.
The most 15N-depleted substances from natural terrestrial environments include nitrous oxide from groundwater undergoing microbial denitrification (δ15N = −55 ‰). Still lower values have been reported for NOx escaping from a nitric acid production facility (δ(15N) = −150 ‰, x(15N) = 0.003 115, and Ar(N) = 14.006 18), and for a commercially available potassium nitrite reagent (δ(15N) = −80 ‰, x(15N) = 0.003 371, and Ar(N) = 14.006 43).
The annotation "g" reflects the fact that a number of samples are known to have atomic weights outside the uncertainties of the standard atomic weight of nitrogen. Many thousands of isotopic analyses of nitrogen have been made since the 1950s; nevertheless, more occurrences of extreme values may be expected as work expands in the fields of contaminant hydrology, biology, and atmospheric chemistry.
Atomic weights of the elements 2009 by M.E. Wieser and T.B. Coplen. Pure Appl. Chem. 2011 (83) 359-396
CIAAW
Nitrogen
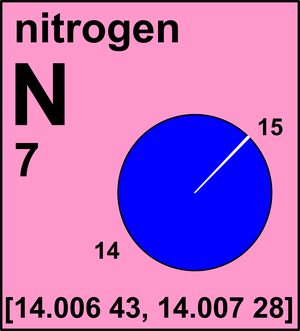
Ar(N) = [14.006 43, 14.007 28] since 2009
The name derives from the Latin nitrum and Greek nitron for "native soda" and genes for "forming".
Nitrogen was discovered by the Scottish physician and chemist Daniel Rutherford in 1772.
Natural variations of nitrogen isotopic composition
Isotopic reference materials of nitrogen.