Platinum
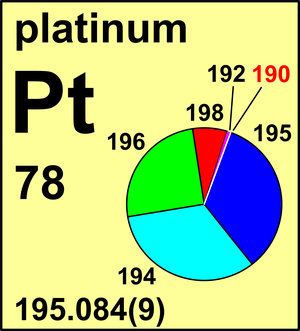
| Isotope | Atomic mass (Da) | Isotopic abundance (amount fraction) |
|---|---|---|
| 190Pt | 189.959 950(5) | 0.000 12(2) |
| 192Pt | 191.961 04(2) | 0.007 82(24) |
| 194Pt | 193.962 683(3) | 0.328 64(410) |
| 195Pt | 194.964 794(3) | 0.337 75(240) |
| 196Pt | 195.964 955(3) | 0.252 11(340) |
| 198Pt | 197.967 90(2) | 0.073 56(130) |
In 1961, the Commission recommended Ar(Pt) = 195.09(3) based on the determinations of the isotopic composition of this element. In 1979, the Commission corrected a small computational error by which Ar(Pt) was amended to 195.08(3). In 2005, the standard atomic weight of platinum was changed to Ar(Pt) = 195.084(9).
The minor isotope, 190Pt, is radioactive with a very long half-life of 6.9(6)×1011 a. It does not affect the atomic weight of platinum even over geological time.
© IUPAC 2003
CIAAW
Platinum
Ar(Pt) = 195.084(9) since 2005
The name derives from the Spanish platina for "silver". In 1735, the Spanish astronomer
Antonio de Ulloa found platinum in Peru, South America. In 1741, the English metallurgist Charles
Wood found platinum from Colombia, South America. In 1750, the English physician William
Brownrigg prepared purified platinum metal.
Isotopic reference materials of platinum.


