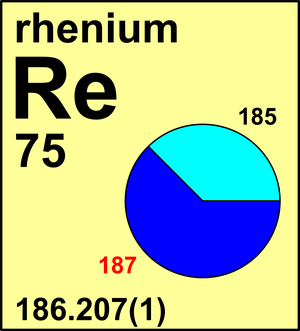
Rhenium
| Isotope | Atomic mass (Da) | Isotopic abundance (amount fraction) |
|---|---|---|
| 185Re | 184.952 958(6) | 0.3740(5) |
| 187Re | 186.955 752(5) | 0.6260(5) |
In 1969, the Commission recommended the atomic weight of Re to be Ar(Re) = 186.2(1) based on the isotopic-abundance measurements. In 1973, the Commission recommended a new value Ar(Re) = 186.207(1) that was based on the superior calibrated measurements. No isotopic composition variations in sources of natural Re have been found thus far. In 1969, after evaluating the uncertainties associated with the mass-spectrometric measurements, the Commission assigned a value of Ar(W) = 183.85(3). For a number of years after that, in the absence of calibrated mass-spectrometric measurements, the Commission was concerned about a discrepancy between the recommended atomic weight and the results of earlier chemical determinations that yielded values of around Ar(W) = 183.90.
187Re is radioactive, decaying to 187Os with a half-life of 4.23(13)×1010 a. Thus, it will take a billion years for the abundance of that isotope to decline by appreciably more than the uncertainty in its current value. However, the anomalies caused in the isotopic composition of some Os occurrences are of geological interest.
© IUPAC 2003
CIAAW
Rhenium
Ar(Re) = 186.207(1) since 1973
The name derives from the Latin rhenus for the Rhine river in Germany. Rhenium was discovered by x-ray spectroscopy
in 1925 by German chemists Walter Noddack, Ida Tacke, and Otto Berg.
Isotopic reference materials of rhenium.