Sulfur
| Isotope | Atomic mass (Da) | Isotopic abundance (amount fraction) |
|---|---|---|
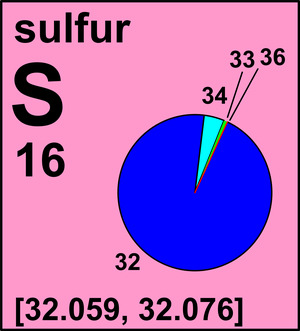
| 32S | 31.972 071 174(9) | [0.9441, 0.9529] |
| 33S | 32.971 458 91(1) | [0.007 29, 0.007 97] |
| 34S | 33.967 8670(3) | [0.0396, 0.0477] |
| 36S | 35.967 081(2) | [0.000 129, 0.000 187] |
Because of natural variations, the uncertainty in the standard atomic weight corresponds to an uncertainty which is more than two orders of magnitude larger than the analytical uncertainties of best measurements. Isotopes of sulfur are fractionated by various chemical, physical, and biological processes. The major variations in the atomic weight of sulfur on earth are caused by kinetic isotope fractionations accompanying microbial oxidation-reduction reactions such as bacterial reduction of aqueous sulfate, in which the residual unreacted substrate is gradually depleted in the lighter isotopes, which react more rapidly. Over geologic time, processes such as these have resulted in major reservoirs of terrestrial sulfur with different atomic weights: oxidized forms such as marine sulfate commonly being heavy in comparison with the bulk earth and the majority of reduced forms such as organic sulfur and sulfide.
Primordial sulfur held in the deep earth and released in some volcanic emissions has a δ(34S) value close to 0 ‰ [Ar(S) = 32.0639]. Seawater sulfate currently has a uniform δ(34S) value of +21.1 ‰ [Ar(S) = 32.0657], though it has been different in the geological past.
The highest value of the atomic weight of sulfur reported in the literature is from sulfate in reduced-sediment pore water undergoing sulfate reduction that had δ(34S) = +135 ‰ [x(34S) = 0.0473 and Ar(S) = 32.075]. The lowest value of the atomic weight of sulfur reported in the literature is from sulfide in an ice-covered sewage treatment lagoon that had δ(34S) value = -55 ‰ [x(34S) = 0.0398 and Ar(S) = 32.059].
The radioactive isotope 35S is produced by cosmic-ray interactions with 40Ar in the atmosphere and decays to 35Cl with a half-life of 87 days. 35S is useful as an environmental tracer in hydrologic studies, both at natural and artificially enriched levels, but its abundance is several orders of magnitude too small to affect Ar(S).
Isotopic compositions of the elements 2013 by J. Meija et al. Pure Appl. Chem. 2016 (88) 293-306
CIAAW
Sulfur
Ar(S) = [32.059, 32.076] since 2009
The name derives from the Latin sulphurium and the Sanskrit sulveri. Sulfur was known as brenne stone
for "combustible stone" from which brim-stone is derived. It was known from prehistoric times and
thought to contain hydrogen and oxygen. In 1809, the French chemists Louis-Joseph Gay-Lussac and
Louis-Jacques Thenard proved the elemental nature of sulfur.
Natural variations of sulfur isotopic composition
Isotopic reference materials of sulfur.


