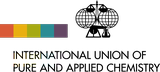Oxygen
| Isotope | Atomic mass (Da) | Isotopic abundance (amount fraction) |
|---|---|---|
| 16O | 15.994 914 619(1) | [0.997 38, 0.997 76] |
| 17O | 16.999 131 757(5) | [0.000 367, 0.000 400] |
| 18O | 17.999 159 613(5) | [0.001 87, 0.002 22] |
Two major sources of oxygen are air and water. Relative isotope-ratio measurements of oxygen in water and many other substances commonly are expressed relative to VSMOW reference material, in which case the δ(18O) value of VSMOW is 0 ‰ by definition. However, two other scales have been used commonly: (1) in studies of atmospheric gases and related topics, atmospheric O2 may be assigned a δ(18O) value of 0 ‰, (2) in studies of marine carbonate deposits and related topics, a specimen of marine carbonate (PDB, Peedee belemnite) may be assigned a δ(18O) value of 0 ‰.
Relating atomic weights to relative isotope-ratio measurements of oxygen may be complicated in principle by the observation that the exponent in the mass-dependent fractionation equation may deviate significantly from one half, and by the fact that relative isotope-ratio measurements generally do not include 17O. Nevertheless, though the value of the 17O exponent may be as high as 0.52 or 0.53 in common substances, the atomic-weight errors caused by these differences are small compared to the uncertainty of the "absolute" measurement of atomic weight. Larger deviations from mass-dependent fractionation of 18O, 17O, and 16O have been observed in minor atmospheric gases such as O3, CO2, N2O, and CO, apparently as a result of non-mass-dependent photochemical reactions. Similar features have been observed in sulfate and nitrate in atmospheric deposition and some types of soils, and it is likely that the number and variety of samples reported to exhibit non-mass-dependent oxygen isotope fractionation will increase rapidly in the future.
Variations in the atomic weight of oxygen in surface water on the earth commonly are correlated with those of hydrogen, as the isotopes of both elements are fractionated by evaporation and condensation. Whereas ocean water has almost constant values of H and O atomic weight worldwide (near that of VSMOW), precipitation varies widely with the lowest values being at high latitudes. Natural variations in the isotopic composition of oxygen have been exploited since the 1950s in studies of the hydrological cycle, biogeochemistry, and paleoclimates.
The highest natural terrestrial δ(18O) value is reported from marine N2O with δ(18O) = +109 ‰, x(18O) = 0.002 218, and Ar(O) = 15.999 76. The lowest natural δ(18O) value is reported from Antarctic precipitation with δ(18O) = −63 ‰, x(18O) = 0.001 875, and Ar(O) = 15.999 04. Given the relatively small uncertainties in the best "absolute" measurements (0.25 ‰) and in typical relative measurements (0.1 ‰ or less), it is evident that the uncertainty of the standard atomic weight of oxygen is dominated by real natural variations.
Atomic weights of the elements 2009 by M.E. Wieser and T.B. Coplen. Pure Appl. Chem. 2011 (83) 359-396
CIAAW
Oxygen
Ar(O) = [15.999 03, 15.999 77] since 2009
The name derives from the Greek oxys for "acid" and genes for "forming" because the French chemist
Antoine-Laurent Lavoisier once thought that oxygen was integral to all acids.
Oxygen was discovered independently by the Swedish pharmacist and chemist Carl-Wilhelm Scheele in 1771, and the English clergyman and
chemist Joseph Priestley in 1774. Scheele's Chemical Treatise on Air and Fire was delayed in publication
until 1777, so Priestley is credited with the discovery because he published first.
Natural variations of oxygen isotopic composition
Isotopic reference materials of oxygen.